Products
RECELL® System
Products
RECELL® System
Providing point-of-care, multi-cell regenerative skin restoration with the RECELL System
The RECELL System is approved by the FDA for the treatment of thermal burn wounds and full-thickness skin defects, and for repigmentation of stable depigmented vitiligo lesions. RECELL harnesses the regenerative properties of a patient’s own skin to create Spray-On Skin™ Cells, delivering a transformative solution at the point-of-care. This breakthrough technology serves as the catalyst for a new treatment paradigm enabling improved clinical outcomes.

Multi-use RECELL GO
Processing Device
Single-use
RECELL GO Cartridge
Providing point-of-care, multi-cell regenerative skin restoration with RECELL System
The RECELL System is approved by the FDA for the treatment of thermal burn wounds and full-thickness skin defects, and for repigmentation of stable depigmented vitiligo lesions. RECELL harnesses the regenerative properties of a patient’s own skin to create Spray-On Skin™ Cells, delivering a transformative solution at the point-of-care. This breakthrough technology serves as the catalyst for a new treatment paradigm enabling improved clinical outcomes.

WOUND HEALING
thermal burn wounds | full-thickness skin defects
REPIGMENTATION
vitiligo patients
Expedite healing at the cellular level
RECELL uses a small piece of the patient’s skin to create a multi-phenotype suspension of Spray-On Skin Cells.1 The application of different cell types stimulates healing and repigmentation throughout the wound bed.2,3
The power of multi-phenotype Spray-On Skin Cells.
Autologous therapy at point-of-care allows for delivery of the patient’s own living cells:
![]() KERATINOCYTES
KERATINOCYTES
regenerate the epidermis4,5
deposit new extraceullular matrix proteins4
produce melanin to allow restoration of natural pigmentation5
TREATING MORE with Less
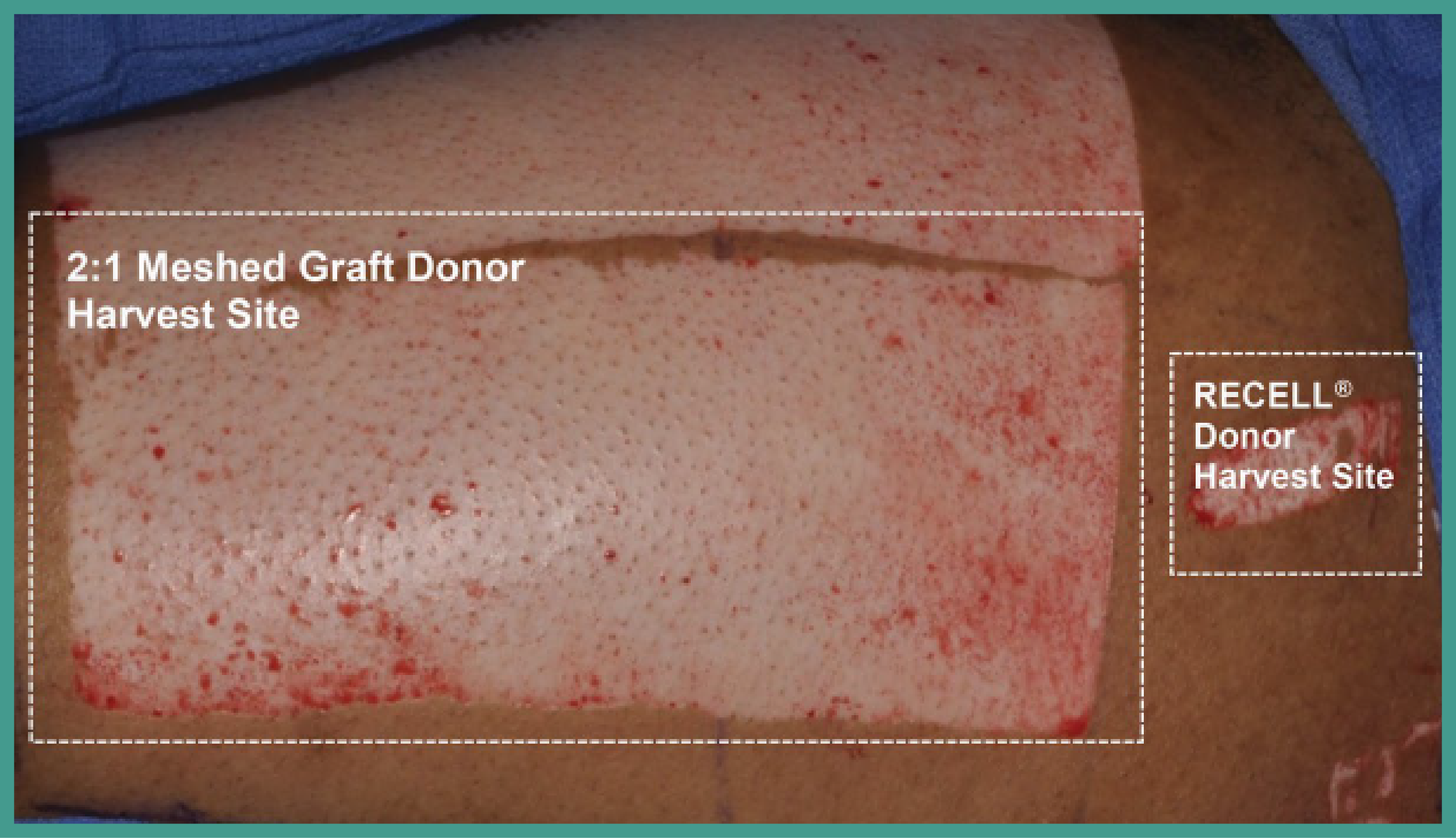
Caring for your patients means more than closing the wound. With RECELL, you can expedite the healing process with early point-of-care treatment of thermal burn wounds and full-thickness skin defects to provide the best possible outcomes for patients. RECELL is designed for donor sparing, significantly less donor skin is required with similar healing outcomes versus conventional autografting.
%
Your Title Goes Here
less Donor
Skin for
DPT BURNS6
%
Your Title Goes Here
LESS DONOR SKIN
FOR FULL-THICKNESS
BURNS WHEN USED
IN COMBINATION WITH
AUTOGRAFT7
%
Your Title Goes Here
LESS DONOR SKIN
FOR FULL-THICKNESS
SKIN DEFECTS
WHEN USED IN COMBINATION
WITH AUTOGRAFT8
STEP-BY-STEP Immediate Cellular Delivery
Cost-effective point-of-care regenerative therapy
in a single procedure8,9

Clinical benefits of RECELL can lead to cost savings.9
Reduced donor skin requirements can result in fewer surgical
procedures for definitive closure, decreased length of stay, and
reduced resource use, translating to potential cost savings.9-12
%
Your Title Goes Here
UP TO 50% REDUCTION
IN DEFINITIVE CLOSURE
PROCEDURES9
%
Your Title Goes Here
UP TO 47% REDUCTION
IN LENGTH OF STAY
FOR BURNS <50% TBSA9
%
Your Title Goes Here
UP TO 39% TOTAL
COST SAVINGS9
HOW THE RECELL SYSTEM WORKS
Explore the RECELL GO® System
PRECISION FOR EVERY PROCEDURE
RECELL GO: Designed for treating wounds up to 1920 cm2
RECELL GO mini™: Designed for treating smaller wounds up to 480 cm2

The proven benefits of RECELL delivered at the touch of a button.
Step 1:
Step 2:

Step 3
Aseptically draw up and apply the RECELL Spray-On Skin Cells.
Patients treated with RECELL Spray-On Skin Cells
versus standard of care for small deep partial-thickness
and full-thickness burns experience shorter hospital
stays to help allow a faster return home.9
Patients treated with RECELL Spray-On Skin™ Cells versus standard of care for small deep partial-thickness and full-thickness burns experience shorter hospital stays to help allow a faster return home.9
Resources that support you and your patients
fREQUENTLY ASKED QUESTIONS
RECELL® is an autologous cell harvesting device used at the point-of-care that processes a small piece of a patient’s own skin to prepare a multi-phenotype suspension of Spray-On Skin™ Cells to treat wounds and support skin regeneration.1-2 The application of different cell types stimulates healing and repigmentation throughout the wound bed.3-4 RECELL can be used for the following types of wounds:
In conjunction with split-thickness skin graft for the treatment of full-thickness:
- Thermal burns
- Traumatic avulsions (e.g. degloving, abrasion)
- Surgical excisions (e.g. fasciotomy, necrotizing infection)
- Surgical resections (e.g. skin cancer removal)
Alone for the treatment of:
- Deep partial-thickness burns
- Wood FM et al. Burns. 2012 Feb;38(1):44-51. 2. Bush KA et al. Int Wound J. 2024 Jun;21(6):e14941. 3. Navarro FA et al. J Burn Care Rehabil. 2001;22:41–6. 4.Navarro et al. J Burn Care Rehabil. 2000;21:513–8.
What are RECELL Spray-On Skin Cells?
RECELL® Spray-On Skin™ Cells are a multi-phenotype cell suspension derived from a patient’s own skin and contain keratinocytes, dermal fibroblasts, and melanocytes to promote healing and repigmentation throughout the wound bed.1-4 Designed for donor sparing, the use of RECELL requires significantly less donor skin than conventional autografting.5
- Wood FM et al. Burns. 2012 Feb;38(1):44-51. 2. Bush KA et al. Int Wound J. 2024 Jun;21(6):e14941. 3. Navarro FA et al. J Burn Care Rehabil. 2001;22:41–6. 4.< Navarro et al. J Burn Care Rehabil. 2000;21:513–8. 5. Instructions for Use. RECELL® Autologous Cell Harvesting Device.
How do RECELL Spray-On Skin Cells contribute to the healing process?
RECELL® Spray-On Skin™ Cells provide fast healing at the cellular level.1 Activated keratinocytes that harness the ‘free-edge’ signal, driving rapid migration and re-epithelialization across the entire wound bed for optimal healing.1
In addition to keratinocytes, RECELL Spray-On Skin Cells contain dermal fibroblasts to deposit extra cellular matrix proteins, and melanocytes to produce melanin and allow restoration of natural pigmentation.2,3
- Bush KA et al. Int Wound J. 2024 Jun;21(6):e14941. 2. Freedberg et al. J Invest Dermatol. 2001;116(5):633-640. 3. Hirobe T. Dermatologica Sinica. 2014;32(4):200-204.
What is RECELL GO and what are its benefits?
RECELL GO® is the next generation, FDA-approved RECELL® System that streamlines preparation of Spray-On Skin™ Cells. It helps reduce medical staff training burden, improve operating room workflow efficiencies, and standardize skin processing to ensure optimal suspension preparation.1
- Bush KA et al. Int Wound J. 2024 Jun;21(6):e14941.
Is RECELL a skin graft?
RECELL® is a regenerative technology and not a skin graft, although its Spray-On Skin™ Cells suspension can be used in a similar manner to a skin graft and offer reduced pain and improved aesthetic outcomes at the RECELL-harvested donor site. 1
- Holmes JH, Molnar JA, Carter JE, et al. A comparative study of the RECELL® device and autologous split-thickness meshed skin graft in the treatment of acute burn injuries. J Burn Care Res. 2018;39(5):694-702.
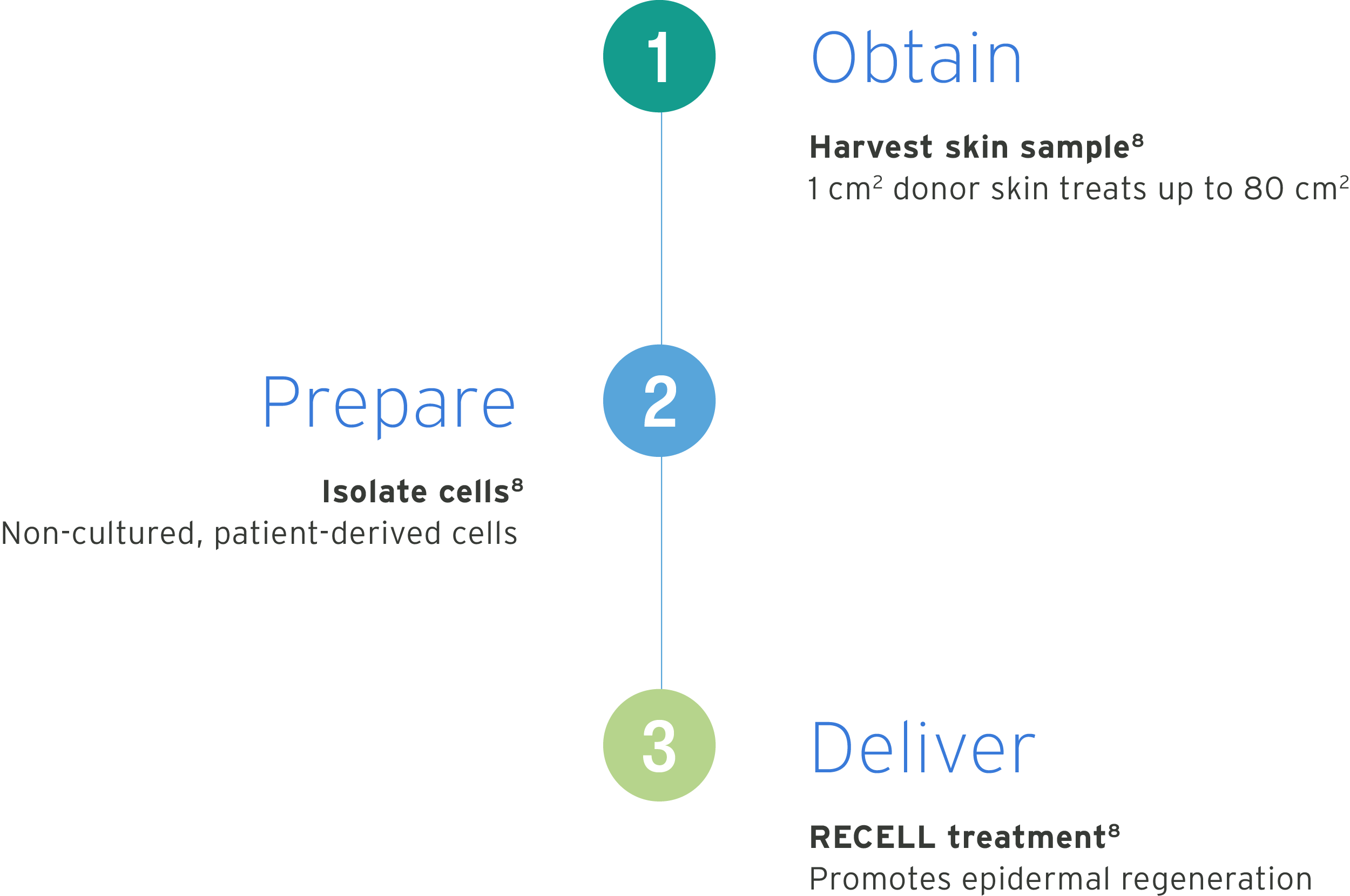
How does RECELL work?
The RECELL® System works by following three steps to prepare RECELL Spray-On Skin™ Cells for wound treatment.
- Obtain: The healthcare provider harvests a small, thin skin sample. 1 cm2 of harvested donor skin treats up to 80 cm.1
- Prepare: Patient-derived cells are isolated using RECELL technology.1
- Deliver: RECELL Spray-On Skin Cells are delivered to the wound site to promote epidermal regeneration.1
- Instructions for Use. RECELL® Autologous Cell Harvesting Device.
Does RECELL reduce the amount of donor skin needed?
The use of RECELL® enables significant donor skin reduction compared to conventional autografting.1 With the RECELL System, 1 cm2 of donor skin can be used to prepare enough Spray-On Skin™ Cells to treat a wound of up to 80 cm2.
- Instructions for Use. RECELL® Autologous Cell Harvesting Device.
Are RECELL Spray-On Skin Cells only for burn wounds?
RECELL® Spray-On Skin™ Cells can be used for a variety of wounds and skin restoration, including:
- Thermal burn wounds: Deep-partial thickness and full-thickness with meshed autograft
- Non-thermal full-thickness wounds with meshed autograft such as traumatic wounds and surgical wounds
Can RECELL be used to treat chronic wounds?
RECELL® can be used to treat complex, difficult-to-heal wounds that have been excised and are ready to graft.
Can RECELL be used for reconstructive plastic surgery wounds?
Yes, RECELL® Spray-On Skin™ Cells can be used on reconstructive plastic surgery wounds. RECELL Spray-On Skin Cells provide the advantage of point-of-care regenerative therapy for wounds, including full-thickness defects and thermal burns, in a single procedure.
How much donor skin does RECELL need?
RECELL® is designed for donor sparing, taking up to 97.5% less donor skin compared to conventional autografting and uses a 1:80 expansion ratio.1 As an example, a 1cm2 donor site can treat an area up to 80x its size.
References: 1. Instructions for Use. RECELL® Autologous Cell Harvesting Device.
HCP Resources
REIMBURSEMENT SUPPORT SERVICES
AVITA Medical is available to assist you with your coding and reimbursement questions. See our coding guide for information about coding for the use of RECELL and RECELL GO:

For general coding, coverage, and reimbursement questions that do not involve specific cases or patient health information, please e-mail us at: hema@avitamedical.com
For more in-depth support with benefits verification, prior authorization, appeals, or case-specific inquiries, please contact AVITA Patient Access Services at:
- E-mail: support@avitapatientaccess.com
- Phone:1-833-674-1688
- Fax: 1-661-749-9430
1. Wood FM, Giles N, Stevenson A, Rea S, Fear M. Characterisation of the cell suspension harvested from the dermal epidermal junction using a ReCell® kit. Burns. Published online November 12, 2011. doi:10.1016/ j.Burns.2011.03.
2. Navarro FA, Stoner ML, Lee HB, et al. Melanocyte repopulation in full-thickness wounds using a cell spray apparatus. J Burn Care Rehabil 2001;22:41–6.
3. Navarro FA, Stoner ML, Park CS, et al. Sprayed keratinocyte suspensions accelerate epidermal coverage in a porcine microwound model. J Burn Care Rehabil 2000;21:513–8.
4. Freedberg I, Tomic-Canic M, Komine M, et al. Keratins and the Keratinocyte Activation Cycle J Invest Dermatol 116:633-640, 2001.
5. Hirobe T. Keratinocytes regulate the function of melanocytes. Dermatol Sin. 2014;32(4):200-204.
6. Holmes JH, Molnar JA, Carter JE, et al. A comparative study of the RECELL® device and autologous split-thickness meshed skin graft in the treatment of acute burn injuries. J Burn Care Res.2018 Aug 17;39(5):694-702.
7. Holmes JH, Molnar JA, Shupp JW, et al. Demonstration of the safety and effectiveness of the RECELL® system combined with split-thickness meshed autografts for the reduction of donor skin to treat mixed-depth burn injuries. Burns.2019;45(4):
8. Instructions for Use. RECELL® Autologous Cell Harvesting Device.
9. Kowal S, Kruger E, Bilir P, et al. Cost effectiveness of the use of autologous cell harvesting device compared to standard of care for treatment of severe burns in the United States. Adv Ther.Published online May 7, 2019. doi: 10.1007/s12325-019-00961-2.
10. Foster K, Bilir P, Kruger E, et al. Cost-effectiveness of RECELL® Autologous Cell Harvesting Device (ACHD) versus STSG for treatment of severe burns in the United States. Presented at the American Burn Association 2018 Annual Meeting, April 2018.
11. Carter JE, Carson JS, Hickerson WL, et al. Length of Stay and Costs with Autologous Skin Cell Suspension Versus Split-Thickness Skin Grafts: Burn Care Data from US Centers [published online ahead of print, 2022 Sep 14]. Adv Ther.2022;10.1007/s12325-022-
12. Carson JS, Carter JE, Hickerson WL, et al. Analysis of real-world length of stay data and costs associated with use of autologous skin cell suspension for the treatment of small burns in U.S. centers [published online ahead of print, 2022 Dec 5]. Burns. 2022; S0305-4179(22)00299-6. doi: 10.1016/j. burns.2022.11.007.
Have questions?
Physicians: stay in touch
Sign Up to
Stay Connected

